Abstract
Introduction: Chimeric Antigen Receptor T-cell therapy (CART) has dramatically improved outcomes for patients (pts) with relapsed/refractory (r/r) DLBCL, but the majority of pts still have poor outcomes due to progressive disease and toxicities. Tools quantifying frailty and comorbidities have not been verified in large patient cohorts. The Cumulative Illness Rating Scale (CIRS) is a comprehensive tool that has been found to predict outcomes in various B cell malignancies. We used a machine learning algorithm to rank the prognostic impact of specific comorbidities measured by CIRS on progression-free survival (PFS) in DLBCL pts undergoing leukapheresis for CART in a multicenter learning cohort (LC; Shouse et al, ASH 2021). In this study, we establish that these comorbidities also predict overall survival (OS) and verify their prognostic significance in a separate validation cohort (VC).
Methods: We conducted a retrospective RWE analysis of pts with r/r DLBCL who underwent leukapheresis for CART at 10 academic centers. CIRS was assessed at the time of T-cell collection and calculated per Salvi (2008). High comorbidity burden was defined using a published cutoff of CIRS score ≥7.
PFS and OS were measured from T-cell collection. Random survival forest (RSF) modeling of PFS and OS was repeatedly applied to random subsets of the LC to determine the most important CIRS categories and comorbidity levels in the presence of known prognostic factors (IPI at diagnosis, concurrent indolent lymphoma, age, ECOG performance status, number of prior therapies, prior transplant, number of medications, cell of origin subtype, complex karyotype, MYC rearrangement by FISH, and MYC+BCL2/BCL6 rearrangement). Cox proportional hazards models were fit to quantify the association between survival and significant pt features. Associations between comorbidities and CART adverse events were evaluated with Fisher's exact test.
Results: We analyzed data from 577 pts. The median CIRS score was 7 (range, 0-25) with 54% (n=309) having CIRS ≥7. The median PFS was 11 months (95% CI: 8 - 15) and OS 30 months (95% CI: 23 - NA), with a median follow-up of 20 months. Although CIRS ≥7 was significantly associated with inferior PFS (HR=1.26) and OS (HR=1.35) in univariable analysis, it did not remain significant in multivariable models.
According to RSF variable importance and node splits, key CIRS categories which adversely impacted OS were respiratory, upper GI, renal, and hepatic. 9% of pts had a score of ≥3 in at least one of the above comorbidities, which we designated Severe4. When accounting for other significant variables, Severe4 was independently associated with inferior PFS (HR=2.45, p<0.001) and OS (HR=2.30, p<0.001) in the LC.
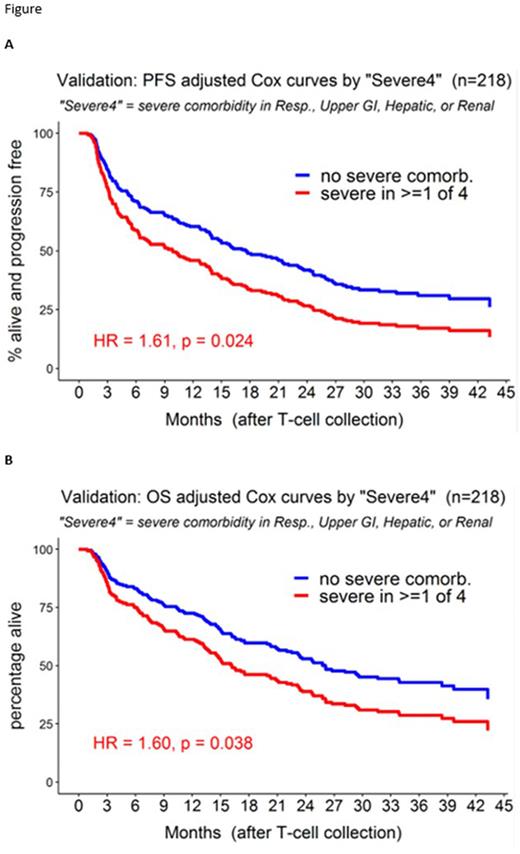
This finding was recapitulated in a single-center VC of 218 patients. In this cohort, median follow-up was 35 months, median age 61 years, median 3 prior therapies, ECOG 0-1 in 74%. As in the LC, axicabtagene ciloleucel was the predominant CART (93%). A CIRS comorbidity score of ≥3 in any of the Severe4 categories was found in 16% of pts. Severe4 remained independently associated with inferior PFS (HR=1.84, p=0.003) and OS (HR=1.82, p=0.007) in the VC (Figure).
Pts with Severe4 also had a higher rate of grade ≥3 CRS (16% vs 6%; p=0.013), while development of grade ≥3 ICANS was associated with CIRS ≥7 (26% vs 12%; p<0.001).
Conclusions: In this large RWE study, we demonstrate that the presence of comorbidity within the Severe4 composite index had prognostic significance for OS in CART recipients for r/r DLBCL. Importantly, Severe4 was validated in a separate cohort. Severe4 is predictive of severe CRS and CIRS ≥7 is predictive of severe ICANS. The underlying mechanism leading to this observation is an area of active investigation. Given these results, CIRS evaluation and Severe4 should be calculated prior to CART in DLBCL to identify those at highest risk for morbidity and mortality and to counsel pts about their expected risk. These results may be product specific given the enrichment of axi-cel patients and may need additional verification with other products.
Disclosures
Shouse:Kite Pharma: Speakers Bureau; Beigene Inc USA: Honoraria. Jaglowski:Gamida: Consultancy; Novartis: Research Funding; Kite: Consultancy, Research Funding; CRISPR Therapeutics: Consultancy. Shadman:Adaptimmune: Consultancy; Atara Biotherapeutic: Consultancy, Research Funding; Fate Therapeutics: Consultancy; Sound Biologics: Consultancy; TG Therapeutics: Consultancy, Research Funding; Morphosys/Incyte: Consultancy, Research Funding; MEI Pharma: Consultancy; Beigene: Consultancy, Research Funding; Innate Pharma: Consultancy; Genentech: Consultancy, Research Funding; AstraZeneca: Consultancy, Research Funding; Abbvie: Consultancy, Research Funding; Kite Pharma: Consultancy; Pharmacyclics: Consultancy, Research Funding; Merck: Consultancy; Regeneron: Consultancy; Mustang Bio: Consultancy, Research Funding; Bristol Myers Squibb: Consultancy, Research Funding; Epi Lilly: Consultancy; Epizyme: Consultancy; Adaptive Biotechnologies: Consultancy; Celgene, a BMS Company: Research Funding; Gilead: Research Funding; Sunesis: Research Funding; Genmab: Research Funding. Patel:Abbvie: Consultancy; Adaptive Therapeutics: Research Funding; Aptevo Therapeutics: Research Funding; AstraZeneca: Consultancy, Research Funding, Speakers Bureau; BiGene: Consultancy; BMS: Consultancy, Research Funding, Speakers Bureau; Caribou Biosciences: Consultancy; Celgene: Consultancy, Research Funding, Speakers Bureau; CRISPR Therapeutics: Research Funding; Curis, Inc: Research Funding; Epizyme: Consultancy, Research Funding; Fate Therapeutics: Research Funding; Genentech/Roche: Consultancy, Research Funding, Speakers Bureau; Kite: Consultancy, Research Funding, Speakers Bureau; Loxo Oncology: Consultancy, Research Funding; Mei Pharma: Consultancy, Research Funding; Morphosys: Consultancy; Nurix: Research Funding; Pharmacyclics/Janssen: Consultancy, Research Funding, Speakers Bureau; Sunesis Pharmaceuticals: Research Funding; TG Therapeutics: Consultancy, Speakers Bureau; Trillium Therapeutics/Pfizer: Consultancy, Research Funding; Velos Bio: Research Funding; Xencor: Consultancy, Research Funding. Stephens:Arqule: Research Funding; Beigene: Consultancy; AbbVie: Consultancy; CSL Behring: Consultancy; Acerta: Research Funding; JUNO: Research Funding; Mingsight: Research Funding; Novartis: Research Funding; AstraZeneca: Consultancy; Celgene: Consultancy; Lilly: Consultancy; Newave: Research Funding; Epizyme: Consultancy; Karyopharm: Research Funding; TG Therapeutics: Consultancy; Genentech: Consultancy. Kamdar:TG Therapeutics: Consultancy, Research Funding; Genentech: Consultancy, Other: DMC, Research Funding; Novartis: Research Funding; Abbvie: Consultancy; AstraZeneca: Consultancy; BMS: Consultancy; Adaptive Technologies: Consultancy; ADC Therapeutics: Consultancy; Beigene: Consultancy; Impact Bio: Consultancy; SeaGen: Speakers Bureau; Celgene: Other: DMC. Hill:Novartis: Consultancy, Honoraria, Research Funding; Kite, a Gilead Company: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel Support, Research Funding; BMS: Consultancy, Honoraria, Research Funding. Karmali:BeiGene: Consultancy, Other: Advisory Board, Research Funding, Speakers Bureau; AstraZeneca: Other: Advisory Board, Speakers Bureau; Morphosys/Incyte: Consultancy, Other: Advisory Board, Speakers Bureau; Calithera: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Advisory Board; Genentech/Roche: Consultancy, Other: Advisory Board; Pharmacyclics: Consultancy, Other: Advisory Board; BMS/Celgene: Consultancy, Research Funding; Takeda: Research Funding; Kite: Consultancy, Other: Advisory Board, Research Funding, Speakers Bureau; Karyopharm: Consultancy; Eusa: Consultancy. Nastoupil:Genentech/Roche, MEI, Takeda: Other: DSMC; ADC Therapeutics, BMS, Caribou Biosciences, Epizyme, Genentech/Roche, Gilead/Kite, Genmab, Janssen, MEI, Morphosys, Novartis, Takeda: Honoraria; BMS, Caribou Biosciences, Epizyme, Genentech, Gilead/Kite, Genmab, Janssen, IGM Biosciences, Novartis, Takeda: Research Funding. Kittai:Abbvie: Consultancy; Astrazeneca: Consultancy, Research Funding; Beigene: Consultancy; Janssen: Consultancy. Danilov:Bayer Oncology: Research Funding; Abbvie: Consultancy, Research Funding; Cyclacel: Research Funding; Genentech: Consultancy; Beigene: Consultancy; Astra Zeneca: Consultancy, Research Funding; GSK: Consultancy; Takeda Oncology: Research Funding; Incyte: Consultancy; Pharmacyclics: Consultancy; Nurix: Consultancy, Research Funding; Bristol-Meyers-Squibb: Consultancy, Research Funding; MEI: Consultancy, Research Funding; Morphosys: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal